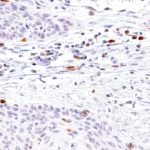
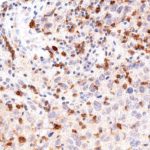
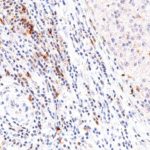
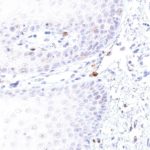
TIGIT at the Immune Checkpoint
A growing number of immune checkpoints develop as targets for anticancer therapy. Cancer immunology studies have shown that cancer cells together with cells of the surrounding microenvironment generate co-inhibitory signals by upregulating the expression of components which suppress the antitumor immune response. The TIGIT pathway interacts with different inhibitory checkpoint pathways. TIGIT provides significant promise for immunotherapy, especially in combination with other immune checkpoint inhibitors.
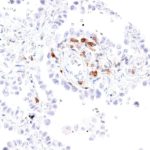
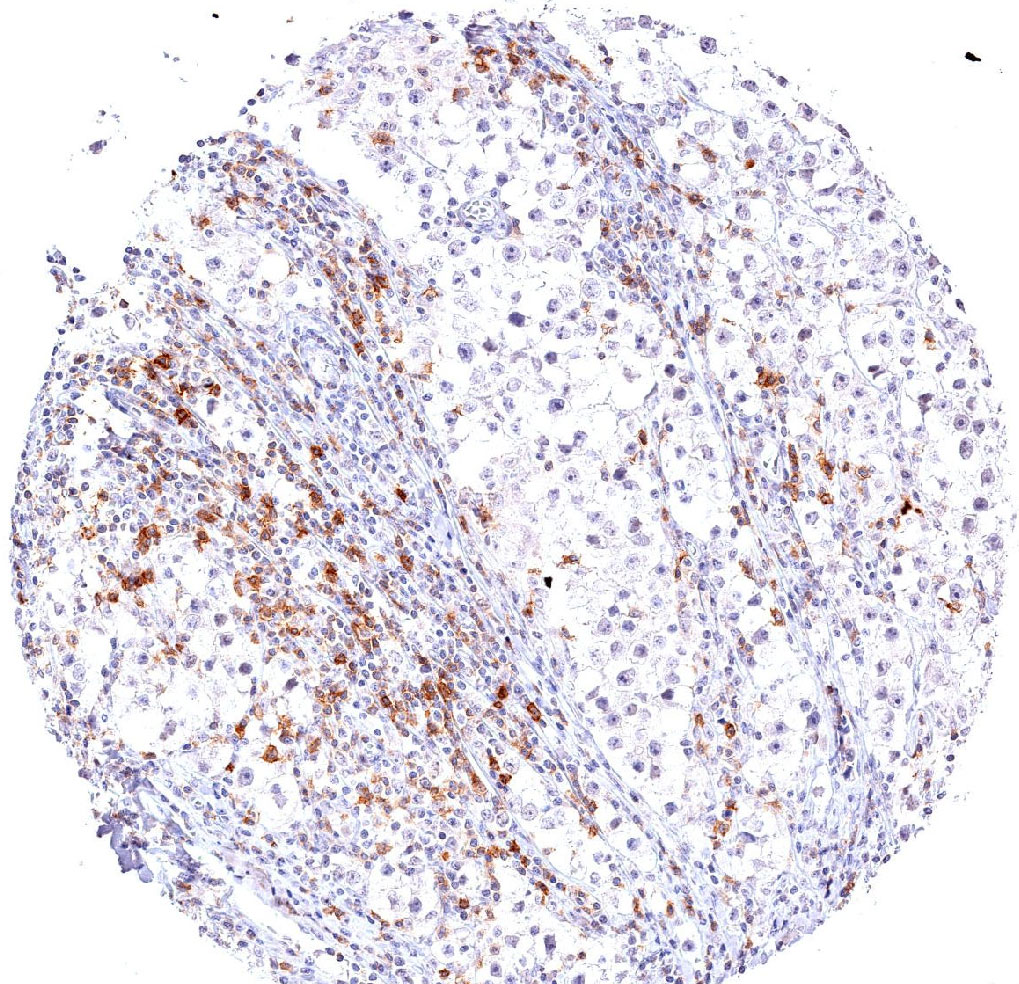
The immunoreceptor TIGIT (T-cell immunoreceptor with Ig and ITIM domains) is a member of the poliovirus receptor (PVR) family. Expression of TIGIT has been reported on NK cells, regulatory T cells, follicular T helper cells, memory CD4+ T cells, and CD8+ T cells, but TIGIT is not expressed on B cells or naive CD4+ T cells. TIGIT acts as an inhibitory immune checkpoint on both T cells and natural killer (NK) cells by a highly complex pathway. Known ligands for TIGIT include CD155 and CD112. Moreover, the TIGIT/CD155/CD112 network also interacts with further checkpoint regulators.
In inflammation and in multiple cancer models T cells have been shown to upregulate TIGIT expression. In several types of cancer, the ligands CD155 and CD112 are also highly expressed on dendritic cells and macrophages. Moreover, TIGIT expression is highly correlated with the expression of other co-inhibitory molecules, including PD-1. In addition to directly inhibiting cytotoxic T-cell activity, TIGIT can stimulate an immunosuppressive microenvironment by influencing other immune cells: For example, TIGIT binds CD155 on the surface of dendritic cells or manipulates NK cell activity. Drugs inhibiting TIGIT activity are currently being developed.
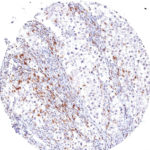
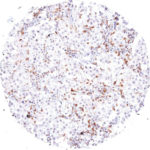
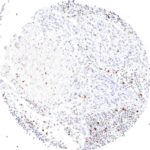
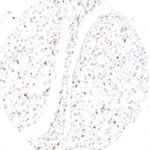
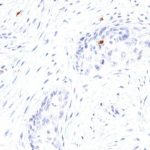
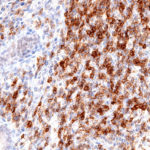
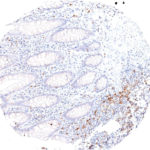
Antibody clone TG1
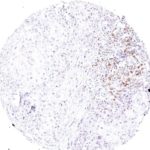
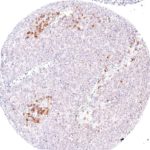
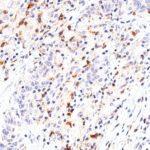
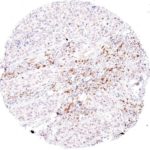
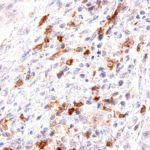
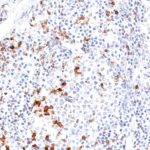
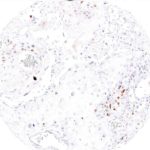
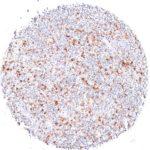
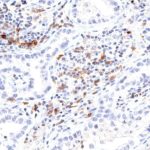
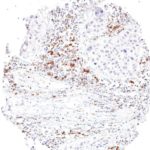
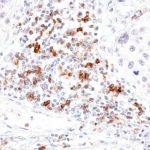
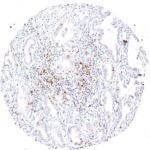
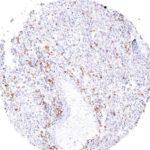
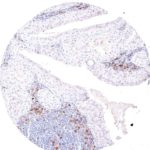
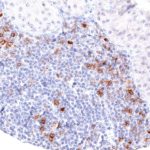
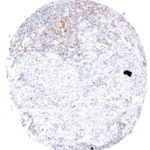
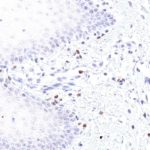
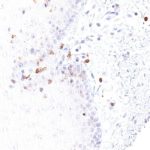
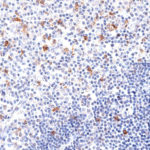
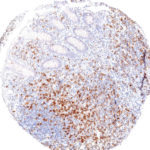
Clone TG1 is the first monoclonal antibody for the immunohistochemical (IHC) detection of TIGIT (T cell immunoreceptor with Ig and ITIM domains) in routine FFPE human tissue specimen. Furthermore TG1 has been validated for the identification of TIGIT positive T-cells infiltrating human tumors with the aim to allow a detection of TIGIT in the tumor microenvironment by IHC. Immunohistochemical (IHC) application of monoclonal antibody TG1 may provide valuable information for clinical research and potential therapeutic interventions specifically targeting the TIGIT-related tumor immunology checkpoint.
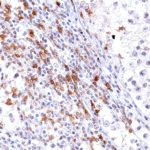
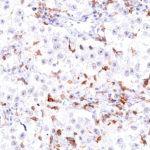
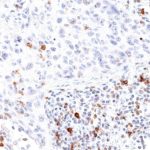
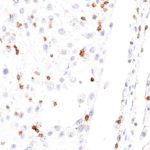
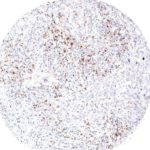
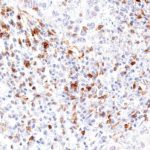
New – clone TG2
The newly developed clone TG2 has a higher affinity to the target protein TIGIT and shows a stronger staining intensity in IHC studies on tonsil when compared to TG1.
- Higher affinity as compared to clone TG1 (tested on tonsil)
- Stronger staining intensity
- Higher Dilutability
1.
Li W et al. Expression of the immune checkpoint receptor TIGIT in Hodgkin’s lymphoma. BMC Cancer 2018, 18: 1209.
doi.org/10.1186/s12885-018-5111-1
2.
Blessin NC et al. Patterns of TIGIT expression in normal lymphatic tissue, inflammation and cancer. Disease Markers 2019, Jan 10;2019:5160565. eCollection 2019.
doi.org/10.1155/2019/5160565
3.
Hinsch A et al. et al. Expression of the immune checkpoint receptor TIGIT in seminoma. Oncol Lett. 2019, 18: 1497–1502.
doi: 10.3892/ol.2019.10428
4.
Scimeca, M. et al. Programmed death ligand 1 expression in prostate cancer cells is associated with deep changes of the tumor inflammatory infiltrate composition. Urol. Oncol. 2019, 37, 297.e19-297.e31.
doi: 10.1016/j.urolonc.2019.02.013
5.
Annibali, O., Bianchi, A., Grifoni, A. et al. A novel scoring system for TIGIT expression in classic Hodgkin lymphoma. Sci Rep 11, 7059, 2021.
doi: 10.1038/s41598-021-86655-8
6.
Murakami D et al. Prognostic value of CD155/TIGIT expression in patients with colorectal cancer. PLoS ONE 17(3), 2022.
doi.org/10.1371/journal.pone.0265908
7.
Niebel D et al. DNA methylation regulates TIGIT expression within the melanoma microenvironment, is prognostic for overall survival, and predicts progression‑free survival in patients treated with anti‑PD‑1 immunotherapy. Clinical Epigenetics 14:50, 2022.
doi.org/10.1186/s13148-022-01270-2