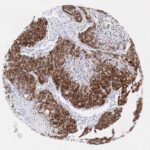
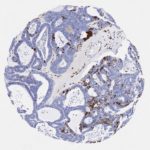
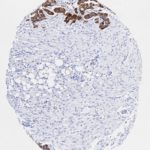
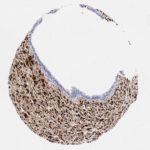
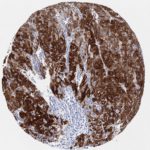
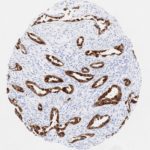
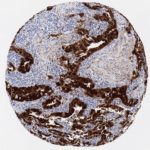
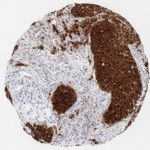
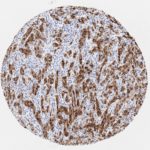
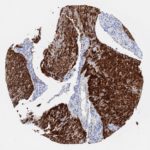
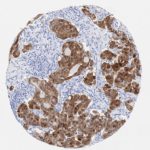
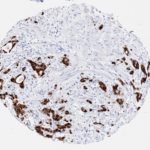
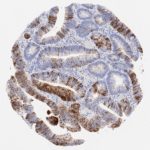
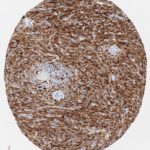
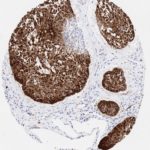
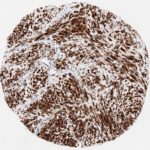
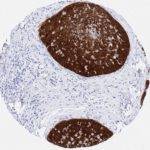
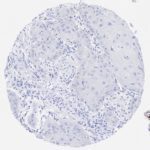
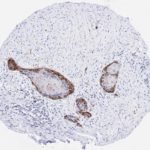
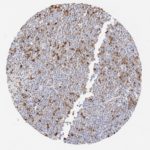
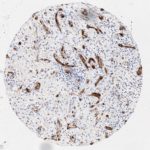
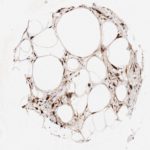
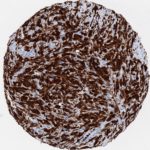
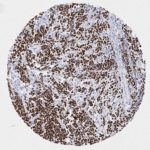
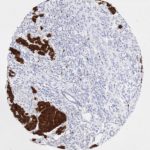
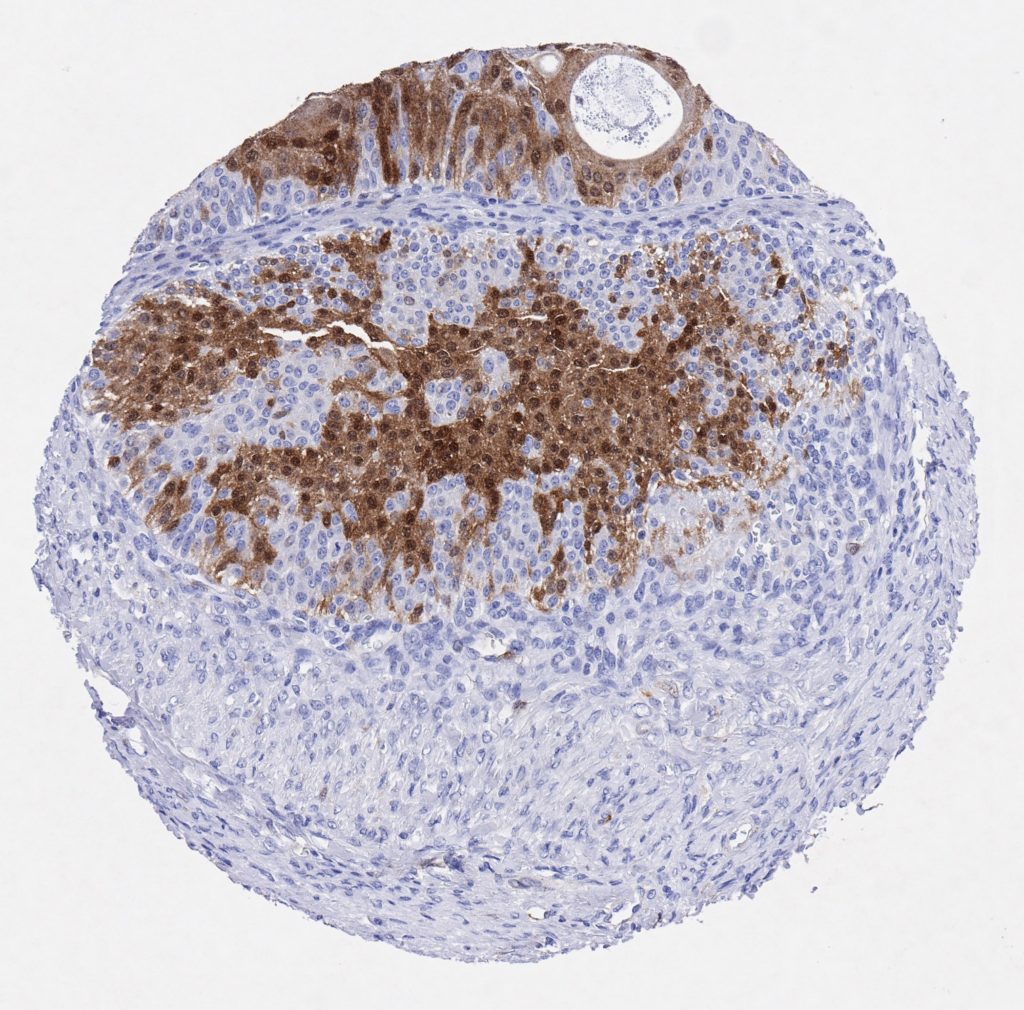
p16 plays an important role in cell cycle regulation. It is the principal member of the Ink4 family of cyclin-dependent kinase (CDK) inhibitors. Binding of p16 inhibits formation of an active CDK4/6 complex and subsequent phosphorylation of retinoblastoma (Rb) protein. Since phospohorylation of Rb protein is a critical step for cell cycle progression from G1 to S phase, p16-binding to the upstream kinase leads to cell cycle arrest. Consequently, p16 is a negative regulator of cell proliferation and thus, a strong tumor suppressor.
Approx. 50% of all human cancers show p16 inactivation, these include head and neck, esophagus, biliary tract, liver, lung, bladder, colon and breast carcinomas; leukemia; lymphomas; and glioblastomas. Moreover, besides downregulation of p16 in cancer, p16 overexpression has been observed in HPV (human papilloma virus)-related tumors, cervical cancer and head and neck squamous carcinomas. The p16-Rb pathway is a target for viral oncoproteins. The E7 oncoprotein from HPV inactivates Rb. Thus, p16 overexpression in HPV-related tumors reflects cell cycle dysregulation by an unsuccessfull attempt to stop cell proliferation.
p16 is an important immunohistochemical (IHC) marker in gynecologic pathology.