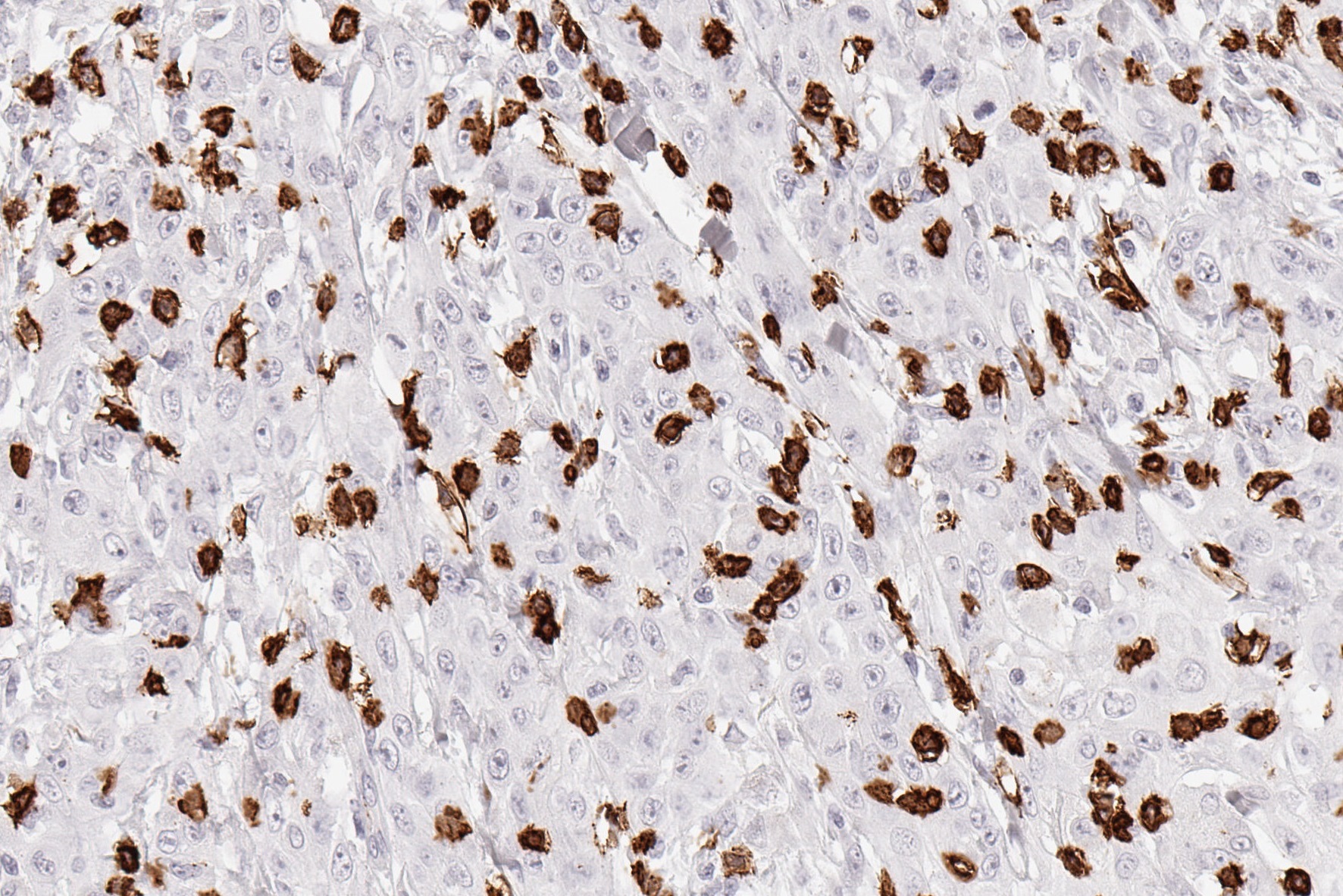
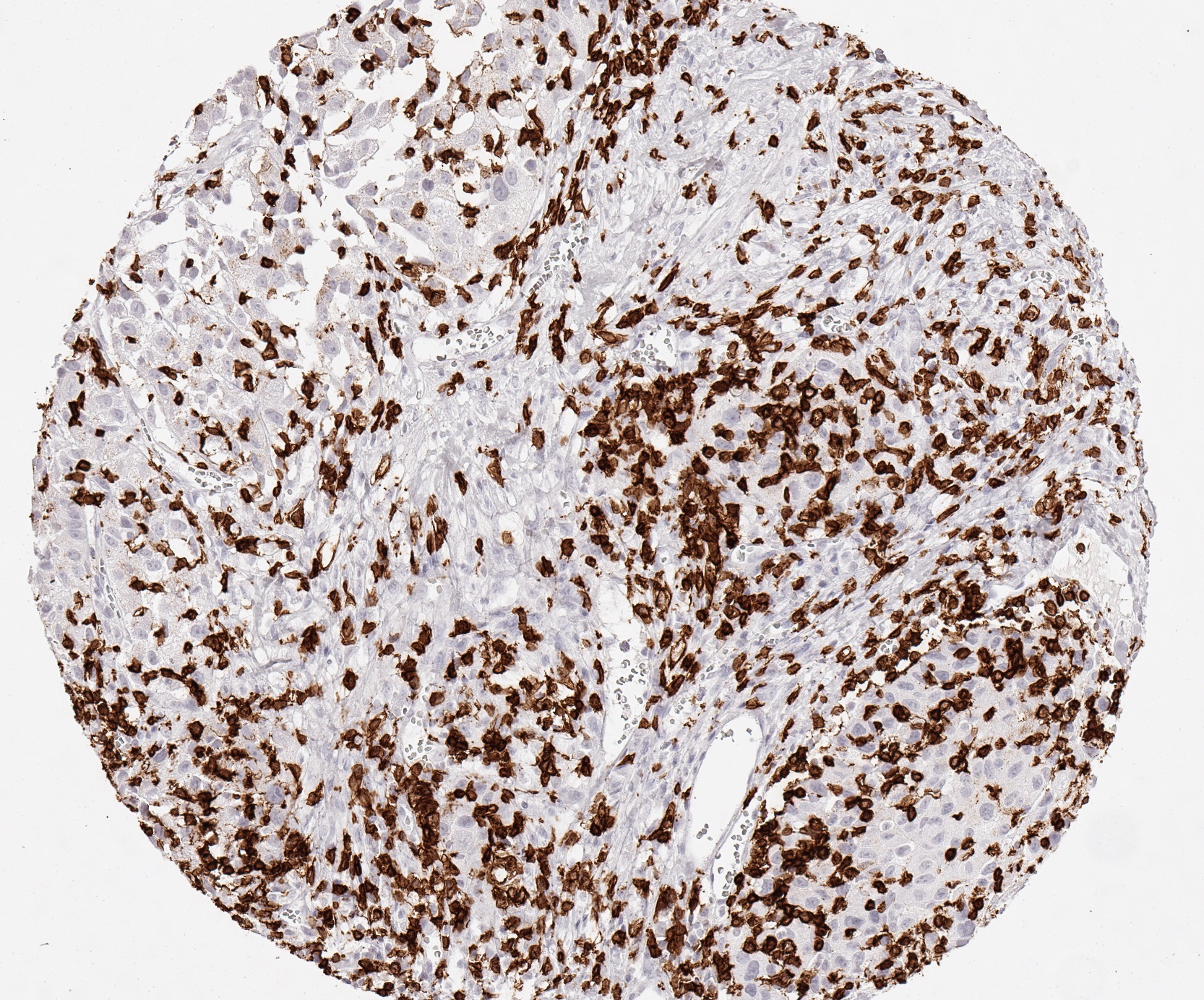
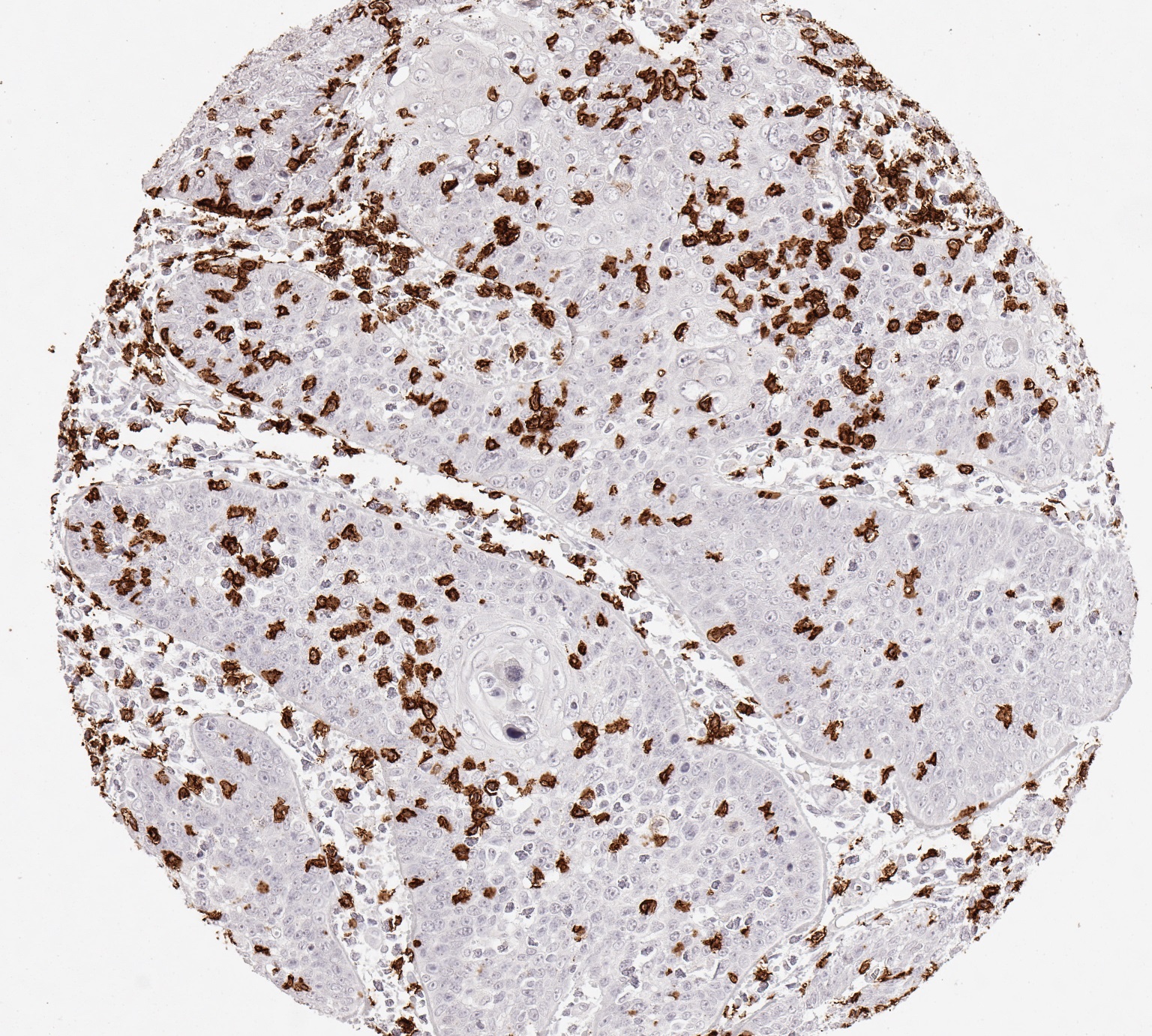
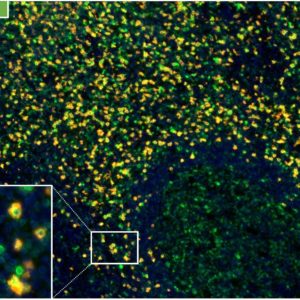
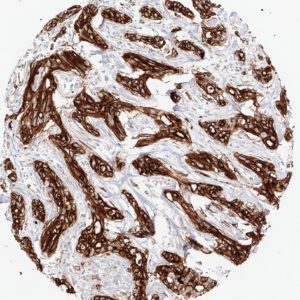
Reaktivität
Clone TC8 has been developed specifically for the immunohistochemical (IHC) detection of CD8 in routine FFPE human tissue specimen. TC8 has been validated for the identification of CD8 positive tumor infiltrating T cells (TILs) with the aim to allow an unequaled specific detection of CD8 in the tumor microenvironment. IHC application of monoclonal antibody TC8 may provide valuable information for clinical research and potential therapeutic interventions targeting the tumor immunology checkpoint.
IHC Protokoll
Staining protocols for anti-human CD8 antibody clone TC8
| Cat.No.: |
DIA-TC8 |
| Isotype: |
Mouse IgG2a/k |
| Specificity: |
Human CD8 |
| Physical State: |
Lyophilized powder |
| Reconstitution: |
DIA-TC8, restore to 500µl. Reconstitute with sterile destilled water by gentle shaking for 10 minutes. |
DAKO – autostainer Link 48
-
Pretreatment buffer: 15 min. / 95°C / pH9
-
Incubation primary antibody: 20 min. / RT (Dilution: 1:200)
-
Link: no
-
HRP (polymer): 20 min. / RT
BOND – Leica Bond RX
- Pretreatment buffer: 15 min / 100°C / pH9
- Incubation primary antibody: 15 min (Dilution 1:200)
- Post Primary: 8 min
- HRP (Polymer): 8 min
Ventana – Discovery Ultra
- Pretreatment buffer: 24 min / 100°C / pH9
- Incubation primary antibody: 60 min / 38°C (Dilution 1:200)
- Secondary antibody: 12 min / 36°C
- HRP (polymer): 12 min
Manual stain with autoclave
- Pretreatment buffer: 121°C / 5 min / pH7,8
- Incubation primary antibody: 60 min / 37°C (Dilution: 1:200)
- Envision HRP rabbit/mouse: 30 min / 37°C
Manual stain with microwave
- Pretreatment buffer: 90 sec, 1000 Watt to cooking, then 15 min / 270 Watt / pH7,8
- Incubation primary antibody: 60 min / 37°C (Dilution: 1:100)
- Envision HRP rabbit/mouse: 30 min / 37°C
2-colour immunoflorescence manual stain
- Position 1: Antibody at pH9
- Position 2: CD8
- Pretreatment buffer: 90 sec, 1000 Watt to cooking, then 15 min / 270 Watt / pH9
- Incubation primary antibody: 30 min / RT (Dilution 1:200)
- HRP (Polymer): 10 min / RT
Referenzen
Specific references for clone TC8
1.
Blessin et al. Patterns of CD112R expression in normal lymphatic tissues, inflammation and cancer. Proceedings: AACR Annual Meeting 2020; April 27-28, 2020 and June 22-24, 2020; Philadelphia, PA, Volume 80, Issue 16 Supplement, pp. 3870.
DOI https://doi.org/10.1158/1538-7445.AM2020-3870
2.
Simon et al. Prognostic role of CD112R, PD-1 and Ki67 expression in CD8+cytotoxic T cells in colorectal cancer. Proceedings: AACR Annual Meeting 2020; April 27-28, 2020 and June 22-24, 2020; Philadelphia, PA, Volume 80, Issue 16 Supplement, pp. 4970.
DOI https://doi.org/10.1158/1538-7445.AM2020-4970
3.
Eichenauer, T. et al. High level of EZH2 expression is linked to high density of CD8-positive T-lymphocytes and an aggressive phenotype in renal cell carcinoma. World J Urol (2020).
DOI https://doi.org/10.1007/s00345-020-03200-4
4.
Fraune, C. et al. MMR Deficiency is Homogeneous in Pancreatic Carcinoma and Associated with High Density of Cd8-Positive Lymphocytes. Ann Surg Oncol 27, 3997–4006 (2020).
DOI https://doi.org/10.1245/s10434-020-08209-y
5.
Blessin et al. Prevalence of CD8+ cytotoxic lymphocytes in human neoplasms. Cellular Oncology 2020, 43: 421-430.
DOI https://doi.org/10.1007/s13402-020-00496-7
General references
- Weynants P et al. Derivation of tumor-specific cytolytic T-cell clones from two lung cancer patients with long survival. Am J Respir Crit Care Med (1999) 159(1):55–62.
- Echchakir H et al. Evidence for in situ expansion of diverse antitumor-specific cytotoxic T lymphocyte clones in a human large cell carcinoma of the lung. Int Immunol (2000) 12(4):537–46.
- Karanikas V et al. High frequency of cytolytic T lymphocytes directed against a tumor-specific mutated antigen detectable with HLA tetramers in the blood of a lung carcinoma patient with long survival. Cancer Res (2001) 61(9):3718–24.
- Piccirillo CA et al. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol (2001) 167(3):1137–40.
- Bossi G et al. The secretory synapse: the secrets of a serial killer. Immunol Rev (2002) 189:152–60.
- Pages F et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med (2005) 353(25):2654–66.
- Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother (2006) 29(3):233–40.
- Kvistborg P et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med (2014) (254):254ra128.
- Tumeh PC et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature (2014) 515(7528):568–71.
- Schumacher TN et al. Neoantigens in cancer immunotherapy. Science (2015) 348(6230):69–74.