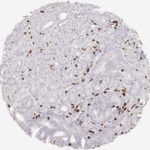
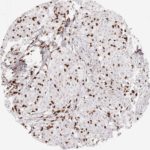
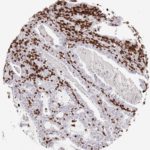
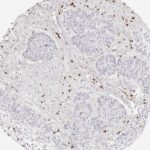
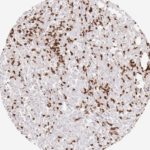
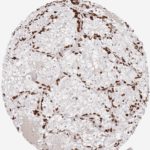
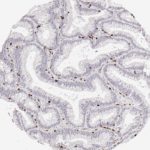
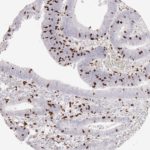
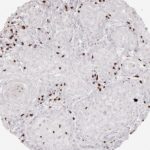
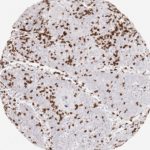
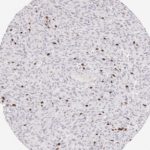
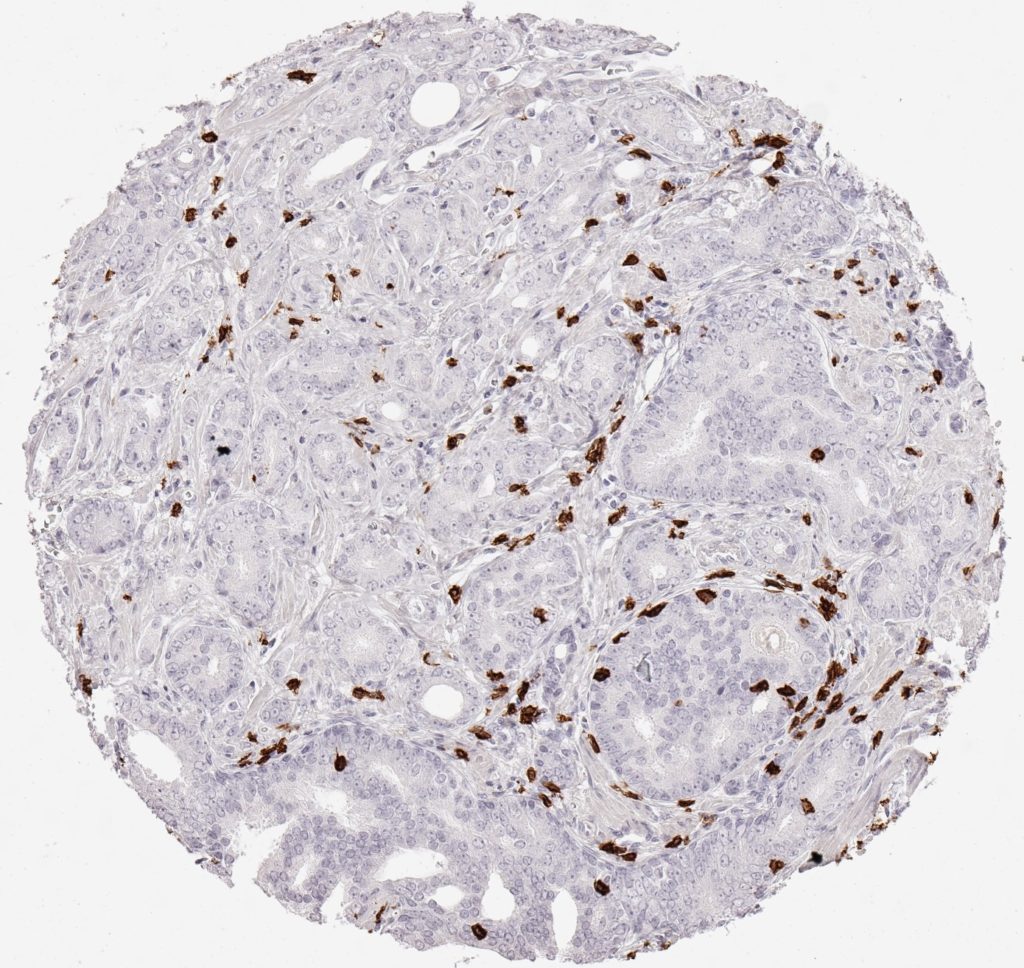
CD8 antibody Clone TC8 has been developed specifically for the immunohistochemical (IHC) detection of CD8 in routine FFPE human tissue specimen. Clone TC8 has been optimized for optimal signal to noise contrast and validated for the identification of CD8+ tumor infiltrating T cells (TILs) with the aim to allow an unequaled specific detection of CD8 in the tumor microenvironment.
CD8+ T cells play a central role for the killing of cancer cells. They have the ability to infiltrate different human tumors and are engaged in the development of a specific tumor microenvironment. Cancer cells have developed mechanisms to successfully evade the antitumor immune response by generating inhibitory signals through upregulation of the expression of immunosuppressive components. Effective blockade of this interaction is considered as a major factor in the development of cancer immunotherapies. Moreover, preexisting CD8+ T cells seem to predict the efficacy of such immune checkpoint therapies.
The T-cell receptor (TCR) recognizes specific antigenic peptides on the surface of cancer and other target cells presented by HLA-I/β2m complexes. Binding to TCR induces a signaling transduction cascade, leading to execution of cytotoxic T lymphocyte (CTL) functions. While CD8+ T cells are directly involved in antitumor cytotoxic responses, the involvement of CD4+ T cells is more indirect, e.g. by their help in priming of CD8+ T cells.
In contrast, inhibitory T-cell receptors such as PD-1, CTLA-4 and TIGIT are activated by the immunosuppressive tumor microenvironment with the aim to inactivate tumor-infiltrating lymphocytes (TILs). The most effective current cancer immunotherapies include immune checkpoint inhibition ICI and block T-cell inhibitory receptors. Moreover, effective blockade immunotherapy appears to be associated with the presence of CD8+ T cells.
Specific references for clone TC8
1.
Blessin et al. Patterns of CD112R expression in normal lymphatic tissues, inflammation and cancer. Proceedings: AACR Annual Meeting 2020; April 27-28, 2020 and June 22-24, 2020; Philadelphia, PA, Volume 80, Issue 16 Supplement, pp. 3870.
DOI https://doi.org/10.1158/1538-7445.AM2020-3870
2.
Simon et al. Prognostic role of CD112R, PD-1 and Ki67 expression in CD8+cytotoxic T cells in colorectal cancer. Proceedings: AACR Annual Meeting 2020; April 27-28, 2020 and June 22-24, 2020; Philadelphia, PA, Volume 80, Issue 16 Supplement, pp. 4970.
DOI https://doi.org/10.1158/1538-7445.AM2020-4970
3.
Eichenauer, T. et al. High level of EZH2 expression is linked to high density of CD8-positive T-lymphocytes and an aggressive phenotype in renal cell carcinoma. World J Urol (2020).
DOI https://doi.org/10.1007/s00345-020-03200-4
4.
Fraune, C. et al. MMR Deficiency is Homogeneous in Pancreatic Carcinoma and Associated with High Density of Cd8-Positive Lymphocytes. Ann Surg Oncol 27, 3997–4006 (2020).
DOI https://doi.org/10.1245/s10434-020-08209-y
5.
Blessin et al. Prevalence of CD8+ cytotoxic lymphocytes in human neoplasms. Cellular Oncology 2020, 43: 421-430.
DOI https://doi.org/10.1007/s13402-020-00496-7